University of California, San Diego | Skaggs School of Pharmacy and Pharmaceutical Sciences
RESEARCH
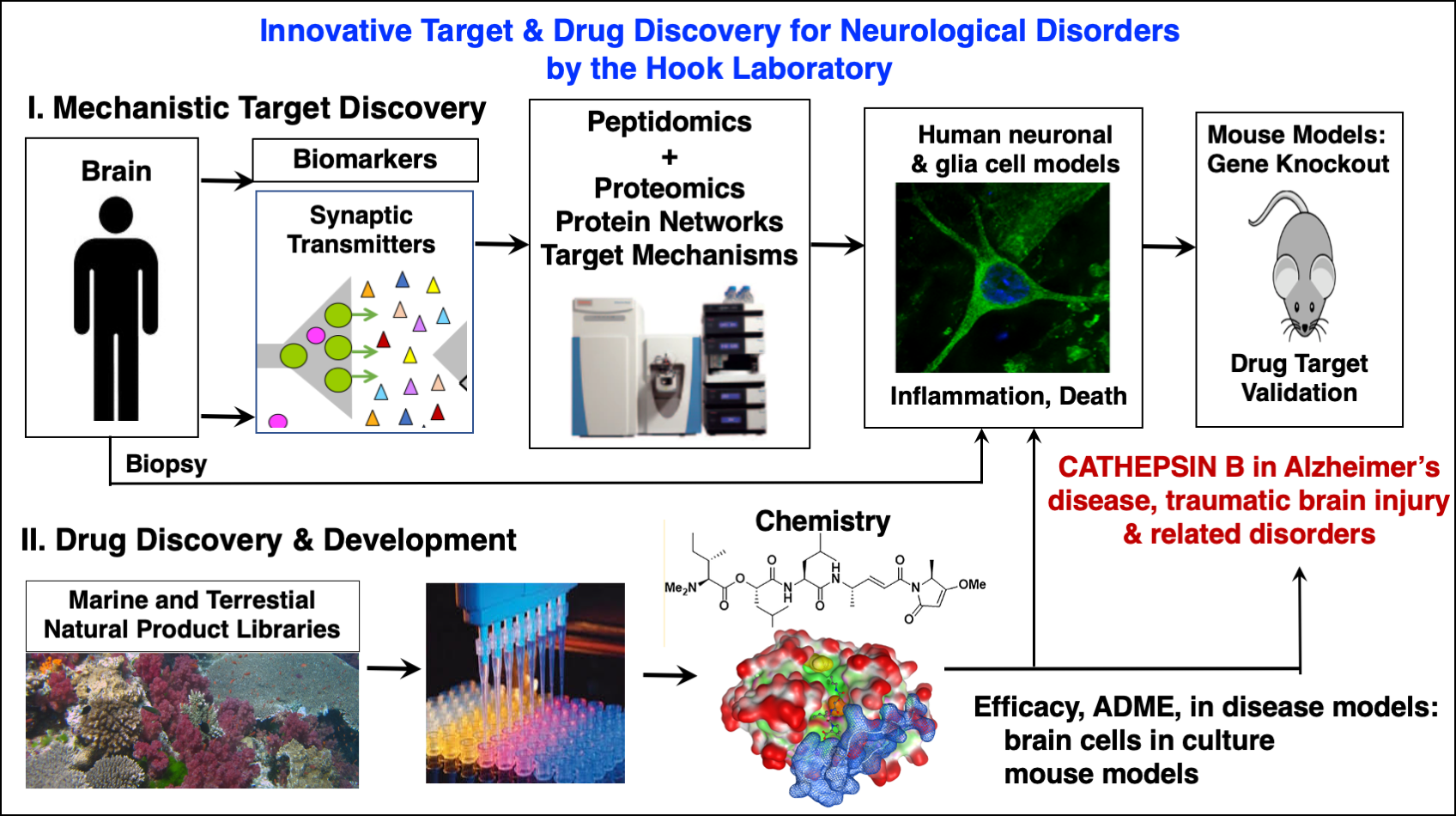
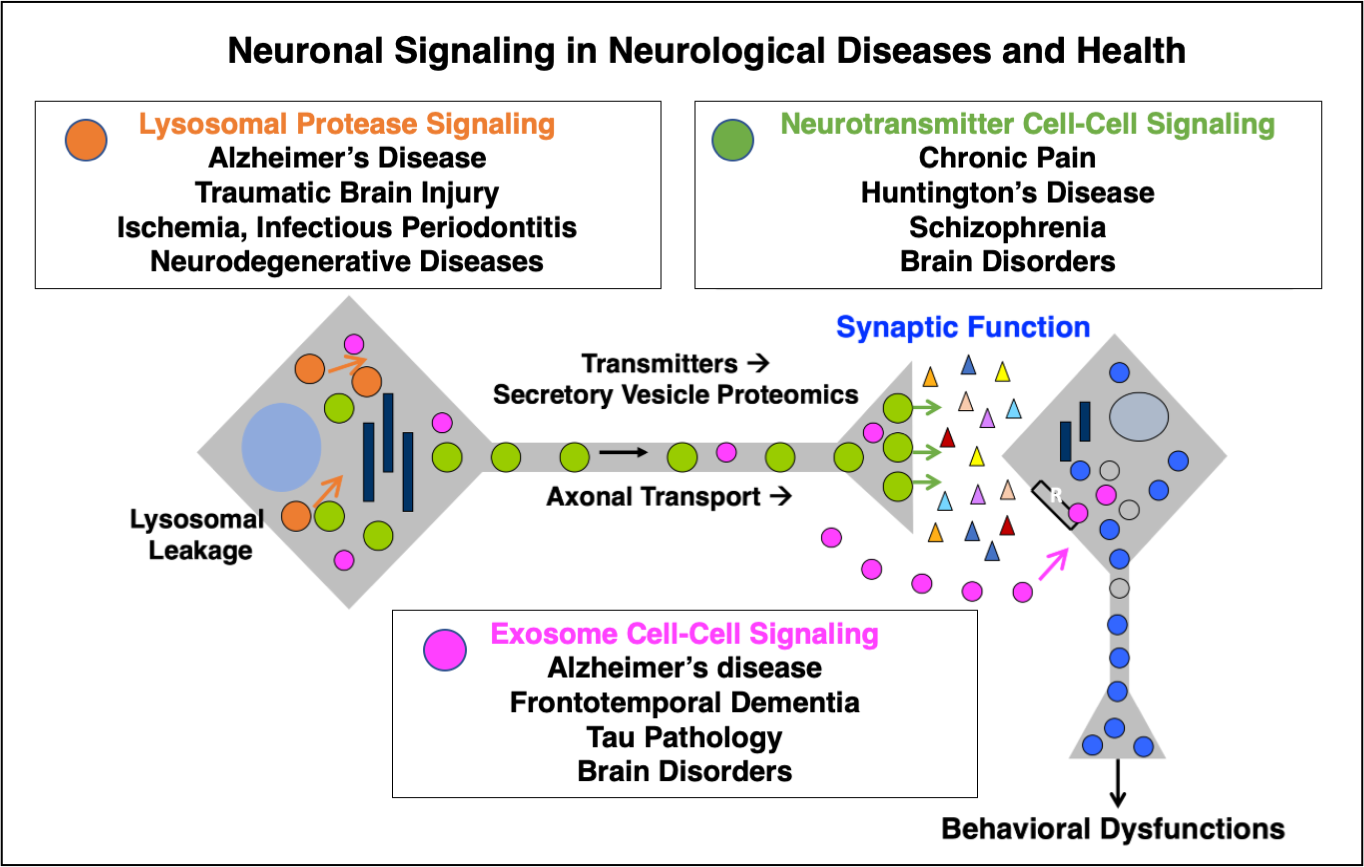
Proteases for Neutrotransmission and Neurodegenerative Diseases: The focus of our research in the Hook Laboratory is to understand how proteases and protease inhibitors are responsible for (1) multi-step proteolytic pathways required for converting precursor proteins into active neuropeptides that function as neurotransmitters, and (2) the protease mechanisms responsible for neurological diseases, including chronic pain, Alzheimer's and Huntington's diseases, and (3) proteomic approaches for elucidation of protease components as potential drug targets and therapeutics. These research disciplines strive to understand the proteolytic controls involved in generating 'beneficial' peptides that promote health, and 'detrimental' peptides in disease. Research findings can lead to development of novel therapeutics for disease and health for medical therapeutics. (A) Proteases for Peptide Neurotransmitter Production. The nervous system requires neuropeptides as neurotransmitters to mediate neurotransmission and a variety of brain functions. The neuropeptide enkephalin and �-endorphin in the brain are required for endgenous pain regulation. The neurotransmitter known as NPY controls feeding behavior and obesity that are regulated by the brain. Galanin is a peptide neurotransmission that regulates cognition and memory. The Hook laboratory strives to define the regulatory proteases and inhibitors that control production of neuropeptides for neurotransmission and brain functions. (B) Protease Mechanisms in Neurodegenerative Diseases: Alzheimer's and Huntington's Diseases. Aberrant proteolytic mechansims are responsible for the development of many neurodegenerative diseases, especially Alzheimer's and Huntington's diseases. Unique patterns of proteolytic processing of mutant gene products in these diseases results in neurotoxic peptide fragments that participate as major pathogenic mechanisms in the disease. Investigations will allow studies of protease inhibitors as therapeutic strategies for treatment of these diseases. (C) Proteomics and Bioinformatic Approaches in Neuroscience Research. In the post-genomic era, it is now critical to understand the function of the gene products in studies of protein function and differential expression profiling of proteins and peptides. We are using interndisciplinary approaches in molecular and cell biology combined with proteomic approaches for protein separation and evaluation of protein expression profiling in the nervous system. We are conducting a proteome study of neurosecretory vesicles, to understand protein components involved in in neurotransmitter release. In addition, proteomics is being utilized to define regulated proteins in drug treatment of the brain, such as morphine, that may specifically relate to the drug's beneficial actions. (D) Proteases Drug Targets for Neurologic Diseases. Elucidation of protease pathways for peptide neurotransmitters and neurotoxic peptides in neurological diseases provides knowledge of new drug targets for therapeutic treatments of pain, Alzheimer's disease, hypertension, and other health conditions. Efforts are underway in optimizing screening assays for identifying small molecules that may serve as candidate drugs to improve health in neurological diseases.
|
Recent Publications:
Hook, V., Yoon, M., Mosier, C., Ito, G., Podvin, S., Head, B.P., Rissman, R., O'Donoghue, A.J., Hook, G. (2020) Cathepsin B in neurodegeneration of Alzheimer's disease, traumatic brain injury, and related brain disorders. Biochim Biophys Acta Proteins Proteom. 1868, 140428.
Boutté, A.M., Hook, V., Thangavelu, B., Sarkis, G.A., Abbatiello, B.N., Hook, G., Jacobsen, J.S., Robertson, C.S., Gilsdorf, J., Yang, Z., Wang, K.K.W., Shear, D.A. (2020) Penetrating Traumatic Brain Injury Triggers Dysregulation of Cathepsin B Protein Levels Independent of Cysteine Protease Activity in Brain and Cerebral Spinal Fluid. J Neurotrauma 37(13),1574-1586.
Podvin, S., Jones, A., Liu, Q., Aulston, B., Ransom, L., Ames, J., Shen, G., Lietz, C.B., Jiang, Z., O'Donoghue, A.J., Winston, C., Ikezu, T., Rissman, R.A., Yuan, S., Hook, V. (2020) Dysregulation of Exosome Cargo by Mutant Tau Expressed in Human-induced Pluripotent Stem Cell (iPSC) Neurons Revealed by Proteomics Analyses. Mol Cell Proteomics 9, 1017-1034.
Yoon, M.C., Solania, A., Jiang, Z., Christy, M.P., Podvin, S., Mosier, C., Lietz, C.B., Ito, G., Gerwick, W.H., Wolan, D.W., Hook, G., O'Donoghue, A.J., Hook, V. (2021) Selective Neutral pH Inhibitor of Cathepsin B Designed Based on Cleavage Preferences at Cytosolic and Lysosomal pH Conditions. ACS Chem Biol. 16, 1628-1643.
Jiang, Z., Lietz, C.B., Podvin, S., Yoon, M.C., Toneff, T., Hook, V., O'Donoghue, A.J. (2021) Differential Neuropeptidomes of Dense Core Secretory Vesicles (DCSV) Produced at Intravesicular and Extracellular pH Conditions by Proteolytic Processing. ACS Chem Neurosci. 12, 2385-2398.
Campeau, A., Mills, R.H., Stevens, T., Rossitto, L.A., Meehan, M., Dorrestein, P., Daly, R., Nguyen, T.T., Gonzalez, D.J., Jeste, D.V., Hook V. (2021) Multi-omics of Human Plasma Reveals Molecular Features of Dysregulated Inflammation and Accelerated Aging in Schizophrenia. Molecular Psychiatry Nature 2021 Nov 5.
Podvin, S., Jones, A., Liu, Q., Aulston, B., Mosier, C., Ames, J., Winston, C., Lietz, C.B., Jiang, Z., O'Donoghue, A.J., Ikezu, T., Rissman, R.A., Yuan, S.H., Hook, V. (2021) Mutant Presenilin 1 Dysregulates Exosomal Proteome Cargo Produced by Human-Induced Pluripotent Stem Cell Neurons. ACS Omega 6(20), 13033-13056.
Boyarko, B., Hook, V. (2021) Human tau isoforms and proteolysis for production of toxic tau fragments in neurodegeneration. Frontiers in Neuroscience 15, 1-19.
Ashhurst, A.S., Tang, A.H., Fajtová, P., Yoon, M.C., Aggarwal, A., Bedding, M.J., Stoye, A., Beretta, L., Pwee, D., Drelich, A., Skinner, D., Li, L., Meek, T.D., McKerrow, J.H., Hook, V., Tseng, C.T., Larance, M., Turville, S., Gerwick, W.H., O'Donoghue, A.J., Payne, R.J. (2021) Potent Anti-SARS-CoV-2 Activity by the Natural Product Gallinamide A and Analogues via Inhibition of Cathepsin L. J Med Chem. 2021 Nov 3